Submissions
Submission Preparation Checklist
As part of the submission process, authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to these guidelines.- The submission has not been previously published, nor is it before another journal for consideration (or an explanation has been provided in the “Cover letter”).
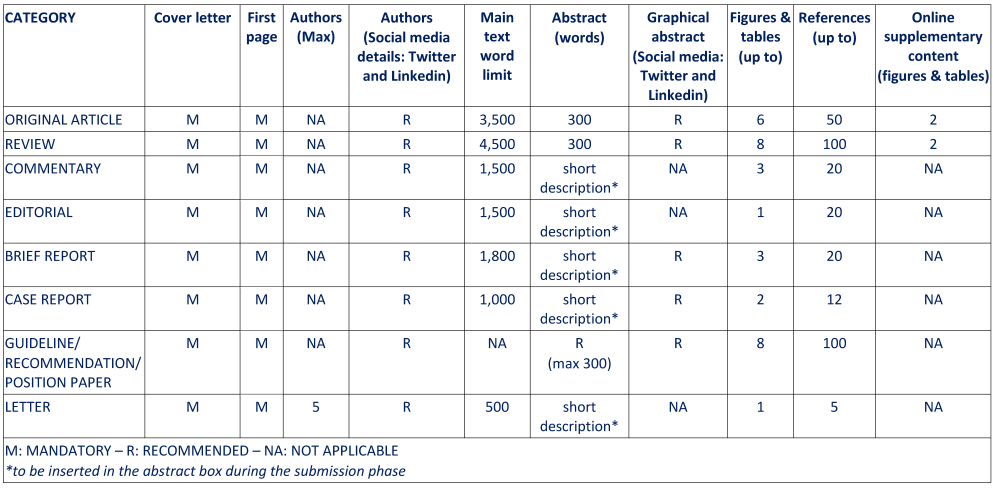
- Cover letter (you should upload your cover letter at “Cover letter” section of the online submission process). It should indicate how the paper satisfies the following criteria: novelty (never published before), originality, impact on the different areas of Transfusion Medicine and Hemostasiology.
- Abstract box: Original article and Review must have an abstract structured in four paragraphs (Background, Material and methods, Results and Discussion), not exceeding 300 words. The abstract should be sufficiently explanatory of the whole paper and should only include text, without any citations and, preferably, without any abbreviations. For all other manuscripts, a short description of the paper is necessary.
- The main manuscript (including title, abstract, text, acknowledgments, conflict of interest disclosure, references, tables and legends for figures) is in Microsoft Word. Use Word “Template”.
-
References adhere to the requirements outlined in the Author Guidelines, for papers with more than six authors, only the first six must be indicated, followed by et al.
Where available, DOI numbers (with relative URLs) for the references have been provided. If DOI is not available, use PMID number. - Images are in .TIFF or .JPEG format, resolution at least 300 dpi (upload in separate files, see Author Guidelines for details. IMPORTANT: maximum dimension for each single file/image is 2 MB). Authors should provide colour images when available.
- The Authorship statement form is properly filled in, signed, saved in .pdf format and uploaded in the next step of the submission process.
- Authors are aware of the publication fee for accepted papers.
Original article
An original article must not exceed 3,500 words (excluding the abstract, references, tables, and illustrations).
The text must be ordered as follows:
- Abstract - Structured in four paragraphs (Background, Material and methods, Results and Discussion), not exceeding 300 words. The abstract should be sufficiently explanatory of the whole paper and should only include text, without any citations and, preferably, without any abbreviations.
- Introduction
- Materials and methods
- Results
- Discussion
- Conclusions
- Acknowledgements (if any)
- Funding and resources (if any)
- Authorship contributions
- Disclosure of conflicts of interest
- References (maximum 50)
- Tables
- Figure legends.
Long articles may need subheadings to clarify some sections, such as Materials and methods, Results and Discussion.
Supplementary data, to be published online only, may include additional information regarding methodology, supplementary figures or tables, or primary data sets. Supplementary material must be submitted with the original manuscript, in order to be peer-reviewed: tables collected at the end of the main text, figures uploaded online. Supplementary content must be cited in the main text as follows: Online Supplementary Content Table S#/Figure S#.
No more than two supplementary tables/figures are allowed.
Review
Review articles are welcomed by the Journal and are generally solicited by the Editor-in-Chief or the Section Editors.
Systematic reviews and meta-analyses must be reported according to PRISMA guidelines.
Authors who wish to submit reviews to the Journal are requested to send a short synopsis of their chosen subject to the Editor-in-Chief, and to indicate the deadline by which they expect to submit their final manuscript. Review articles should focus on recent scientific or clinical advances in an area of broad interest to those in the field of Transfusion Medicine and Hemostasiology. These articles, besides summarising the state-of-the-art, should critically discuss the importance and novelty of the data, and how the findings of the papers reviewed are expected to influence diagnostic processes and treatments.
All Reviews, including those solicited by the Editors, are rigorously peer reviewed before a final publication decision is made.
Manuscripts must not exceed 4,500 words (excluding the abstract, references, tables, and illustrations). The paper must include an abstract of a maximum 300 words; it should be sufficiently explanatory of the whole manuscript and include text only, without any citations and, preferably, without any abbreviations. A maximum of 100 references is recommended.
No more than 8 tables/figures are allowed.
Supplementary data, to be published online only, may include additional information regarding methodology, supplementary figures or tables, or primary data sets. The supplementary material must be submitted with the manuscript, in order to be peer-reviewed: tables collected at the end of the main text, figures uploaded online. Supplementary content must be cited in the main text as follows: Online Supplementary Content Table S#/Figure S#.
No more than 2 supplementary tables/figures are allowed.
Commentary
Commentaries are opinions on papers recently published in Blood Transfusion, or on novel concepts and developments in Transfusion Medicine and Hemostasiology. Commentaries are usually invited articles, but proposals are welcome and should be addressed to the Editorial Office. They have a free structure and contain no more than 1,500 words (excluding references).
A maximum of 20 references is recommended. No more than 3 tables/figures are allowed.
A short description of the paper must be inserted in the abstract box during the submission phase.
Editorial
Editorials that comment on articles published in Blood Transfusion are usually by invitation, and are published in the same issue as the commented paper.
Submissions of Editorials by recognised experts on relevant topics will be considered.
These articles have a free structure and contain no more than 1,500 words (excluding references). A maximum of 20 references is recommended. 1 table/figure is recommended.
A short description of the paper must be inserted in the abstract box during the submission phase.
Brief report
Brief Reports are focused on new findings on a specific and limited topic. They should contain a maximum of 1,800 words (excluding references).
The text must be ordered as follows:
- Introduction
- Materials and methods
- Results
- Discussion
- Conclusion
- Acknowledgements (if any)
- Authorship contributions
- Disclosure of conflicts of interest
- References (a maximum of 20 references is allowed)
- Tables
- Figure legends
No more than 3 tables/figures are allowed.
A short description of the paper must be inserted in the abstract box during the submission phase.
Case report
The submission of case reports will be considered only if the report includes novel scientific material or is of extraordinary clinical interest.
A case report should not exceed 1,000 words (excluding references).
The text must be ordered as follows:
- Introduction
- Case report with results
- Discussion
- Acknowledgements (if any)
- Authorship contributions
- Disclosure of conflicts of interest
- References (a maximum of 12 references is allowed)
- Tables
- Figure legends
No more than 2 tables/figures are allowed.
A short description of the paper must be inserted in the abstract box during the submission phase.
Recommendations
Authors are invited to indicate the methodology used for the preparation of the guideline/recommendations/position paper, or explain why no standard methodology was used. Useful information on reporting guidelines can be found in the ICMJE recommendations and in the EQUATOR Network (www.equator-network.org /home/).
A maximum of 100 references is recommended.
Guidelines
Authors are invited to indicate the methodology used for the preparation of the guideline/recommendations, or explain why no standard methodology was used. Useful information on reporting guidelines can be found in the ICMJE recommendations and in the EQUATOR Network (www.equator-network.org /home/).
A maximum of 100 references is recommended.
Position paper
Authors are invited to indicate the methodology used for the preparation of the guideline/recommendations, or explain why no standard methodology was used. Useful information on reporting guidelines can be found in the ICMJE recommendations and in the EQUATOR Network (www.equator-network.org /home/).
A maximum of 100 references is recommended.
Letter to the Editor
Letters are short, relevant comments that should further readers understanding of articles recently published in Blood Transfusion. Preferably, letters should not exceed 500 words of text, 1 table or figure, and 5 essential references: they should be signed by no more than five Authors.
A short description of the paper must be inserted in the abstract box during the submission phase.
Copyright Notice
AUTHORSHIP STATEMENT FORM
Authorship responsibility
(1) All Authors participated sufficiently in the intellectual content, analysis of data (if applicable) and writing of the article, as defined by the criteria for authorship by the International Committee of Medical Journal Editors (http://www.icmje.org/). (2) The corresponding Author certifies that the definitive version of the manuscript has been approved by all co-Authors, as well as, by the Director of the Institute or Department where the work has been carried out. (3) All persons who have made substantial contributions to the work reported in this manuscript (e.g., data collection, writing or editing assistance) but who do not fulfil the authorship criteria are named along with their specific contributions as an acknowledgement in the manuscript. The corresponding Author certifies that all persons named in the acknowledgement section have provided written permission to be named. (4) All Authors have reviewed the final version of the article and approve it for publication. (5) Authors must state that the article submitted has not been previously published, and is not under consideration or accepted for publication (in whole or in part) elsewhere nor have the Authors assigned any right or interest in the article to any third party. (6) Written permission from the Authors to reproduce any material with copyright elsewhere has been obtained prior to submission. (7) Authors must specify that consent has been obtained from patients taking part in the investigations or, in the case of paediatric patients, from the guardian/s and that they have obtained written releases from patients whose names or photographs are submitted as part of the article. (8) For reports containing original data, the corresponding Author should have full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis. (9) Any manuscript concerned with human subjects, medical records, or human tissue that is submitted to “Blood Transfusion” should comply with the principles stated in the Declaration of Helsinki “Ethical Principles for Medical Research Involving ‘Human Subjects”, adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964, and as amended most recently by the 64th World Medical Assembly, Fortaleza, Brazil, October 2013. If the study involves human or animal subjects or records of human patients ethical approval MUST have been obtained. The corresponding Author must state whether ethical approval was given, by whom and the relevant Judgement’s reference number. The manuscript should contain a statement that the work has been approved by the appropriate Ethical Committee related to the institution(s) in which the work was performed, and that subjects gave informed consent to the work. “Blood Transfusion” requires institutional Ethics Committee approval for all human studies. For retrospective studies on patients’ records either a statement of approval or a statement of exemption from the Committee is required. Ref N° of the Ethical Committee Approval must be indicated in the Authorship Statement Form. The study was approved by the Institutional Ethical Committee (the Name of the Institution must be indicated in the Authorship Statement Form) and this is clearly stated in the Methods section of the article. This statement should also be provided upon submission of the manuscript. Studies involving experiments with animals must state that their care was in accordance with institution guidelines and relevant national laws. (10) The article contains no libellous or unlawful statements, does not infringe the privacy of others, or contains material or instructions that might cause harm or injury. The corresponding Author shall indemnify and hold the Editors and its agents and licensees harmless from any damages, costs, and expenses (including reasonable attorney’s fees and costs of settlement) resulting by reason of any claim, action, or proceeding finally sustained or settled inconsistent with the foregoing warranties and representations. (11) Suspected plagiarism is handled in accordance with the COPE flowcharts (http://publicationethics.org/resources/flowcharts).
Every Author or co-Author of any article published in this Journal is solely responsible for the contents of the article, for the statement made in their paper and for the material sent. Every Author or co-Author needs to fully comply with the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation): «Article 9. Processing of personal data revealing racial or ethnic origin, political opinions, religious or philosophical beliefs, or trade union membership, and the processing of genetic data, biometric data for the purpose of uniquely identifying a natural person, data concerning health or data concerning a natural person's sex life or sexual orientation shall be prohibited».
Privacy policy statement. Information pursuant to Article 13 of the European Data Protection Regulation no. 2016/679 (GDPR)
The personal data indicated above and provided by you will be processed as part of the registration operation on the site and publication of editorial content and will not be disclosed to third parties, with the exception of subjects who are responsible for managing the purpose.
The data will be kept according to the criterion of necessity or until request for cancellation.
The provision of data for this purpose is optional. However, in case of refusal to provide data or to allow their processing it will not be possible to pursue the purposes described. The legal basis of the processing is the consent of the interested party.
The data controller is SIMTIPRO Srl with registered office in Milan Via Desiderio n. 21 cap 20131 (Italy).
In relation to the processing of data concerning you, you may contact the Data Controller to exercise your rights pursuant to articles 15 to 22 of the GDPR by writing to privacy@simtipro.it.
A complaint may be lodged in relation to the processing with the competent Authority:
Garante sulla Protezione dei Dati personali
E-mail: garante@gpdp.it.
Financial disclosure. All Authors must state any information that may be perceived as potential conflict of interest as stated by the ICMJE recommendations. All Authors must disclose all their affiliations including any relevant personal or institutional financial involvement (employment by an industrial concern, consultancies, honoraria, speakers bureau, stock ownership or options, expert testimony, grants received or pending, membership on a standing advisory council or committee, a seat on the board of directors, or being publicly associated with a company or its products, royalties, donation of medical equipment, etc.) with any organization that to any Author’s knowledge has a direct interest, particularly a financial interest, in the subject matter or materials discussed. This declaration will be treated by the Editor as confidential while the paper is under review, and will not be made known to Reviewers. Please indicate on the Authorship Statement Form whether or not you have or may have such a conflict of interest regarding the content of this article its nature of it.
Copyright transfer agreement. (a) Authors assign to “Blood Transfusion”, all copyright in and to the article, including but not limited to the right to publish, republish, transmit, sell, distribute and otherwise use the article in whole or in part, in electronic and print editions of the Journal and in derivative works throughout the world, in all languages and in all media of expression now known or later developed, and to license or permit others to do so. (b) The Authors retain all proprietary rights, other than copyright, such as patent rights. The Authors retain the right to reuse any portion of the work, without charge, in personal compilations or other publications consisting solely of the Author(s’) own works, including the Author(s’) personal web home page, and to make copies of all or part of the Work for the Author(s’) use for lecture or classroom purposes. The corresponding Author declares that any person named as co-Author of the article is aware of the submission and has agreed to being so named. The corresponding Author accepts responsibility for releasing this material on behalf of any and all co-Authors. The corresponding Author declares that statements and opinions given in the article are the expression of the Authors. Responsibility for the content of the article rests upon the Authors.
BT manuscripts are published under CC-BY-NC-ND (Creative Commons Attribution-Non-Commercial-NoDerivatives 4.0 International) license. The articles can be used by giving appropriate credit and mentioning the license, but only for non-commercial purposes and in the original version.
For further information: https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en.